Background
People with sickle cell disease (SCD) have exceptionally high rates of maternal morbidity, including increased risk for venous thromboembolic events (VTE). Data from the National Inpatient Sample shows that VTE risk in hospitalized Black pregnant people with SCD is 14 times that of unaffected Black and non-Black pregnant people. 1 There is little disease-specific data to inform anticoagulation (AC) use during pregnancy. 2 The purpose of this study is to describe VTE events in a SCD pregnancy cohort and to identify risk factors for VTE during pregnancy and compare maternal-fetal outcomes for subjects with or without VTE in pregnancy.
Methods
The Mount Sinai Hospital (MSH) Research Ethics Board and The Johns Hopkins Hospital (JHH) Institutional Review Board approved this retrospective study of pregnant people with SCD of ≥ 20 weeks gestation cared for at MSH, Toronto (1990-2017) or JHH (2000-2021). At MSH and JHH, all pregnant people with SCD and VTE history are prescribed antenatal prophylactic LMWH and for 6-weeks postpartum. At MSH, but not JHH, all receive AC prophylaxis for 6-weeks postpartum. At both centers, hospitalized pregnant people receive prophylactic AC.
We stratified subjects by VTE occurrence during pregnancy. First, we described the cohort with VTE during pregnancy, including subject characteristics, VTE recurrence and pregnancy outcomes. Second, we compared cases with a first pregnancy with VTE to controls without VTE. We excluded repeat pregnancies in both groups.
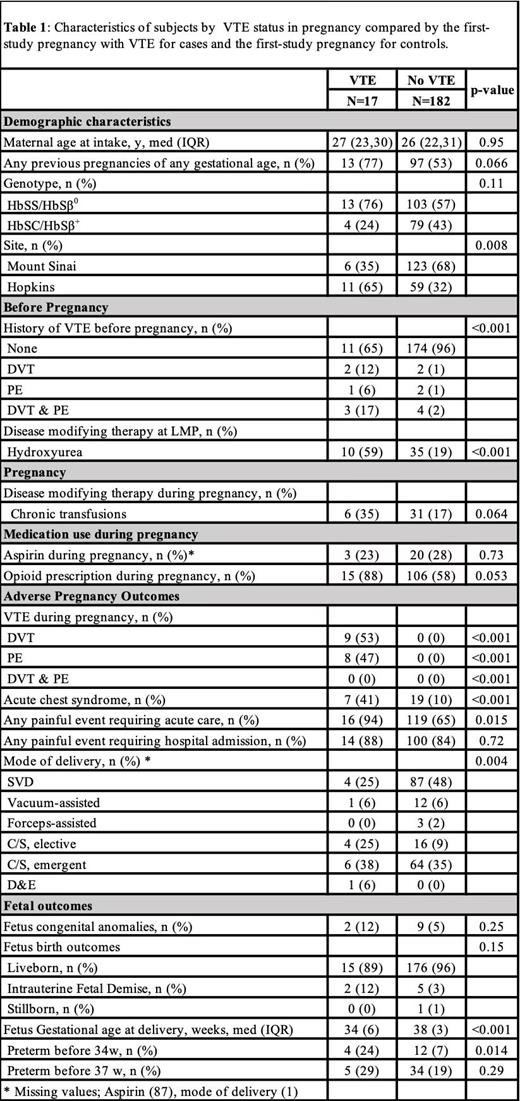
We compared cases and controls by SCD characteristics, medication use before pregnancy, and pregnancy outcomes. Painful events were defined as pain requiring acute care or hospital admission. Chronic transfusion therapy (CTT) was defined as pregnancies treated with scheduled, prophylactic transfusion; opioid use was defined as receiving an outpatient opioid prescription during pregnancy. We used the Chi-squared test for categorical variables and Wilcoxon signed-rank tests for continuous variables. A p-value of <0.05 was considered significant.
Results
There were 290 singleton pregnancies in 199 subjects. Half of the pregnancies were first pregnancies (142/290), with fewer second (51/290), third (2/290), fourth (3/290) or fifth (1/290) pregnancies. The cohort comprised of 58% subjects with HbSS/HbSβ 0, while 42% had HbSC/HbSβ+ genotype (Table 1).
VTE occurred in 7% of pregnancies (20/290) including deep vein thrombosis (DVT, N=9), pulmonary embolism (PE, N=8), DVT+PE (N=3). Median age was 27 years (IQR 23,30). Before pregnancy, most VTE subjects received hydroxyurea (N=10). During pregnancy, six received CTT. Fourteen subjects had VTE in a single pregnancy; three had VTE recurrence in subsequent pregnancies. All three had HbSS/HbSβ 0. Two subjects had VTE history outside of pregnancy. One had DVT in two consecutive pregnancies and was prescribed AC in both pregnancies due to history of VTE. VTE occurred in first (N=4), second (N=8), third (N=4), fourth (N=1) and fifth (N=4) pregnancies. Most VTE occurred at JHH (11 JHH vs 6 MSH, p=0.008).
To investigate SCD-specific risk factors for VTE in pregnancy, we compared characteristics in VTE subjects (N=17) to no-VTE subjects (N=182) by first study pregnancy with an event or first study pregnancy while enrolled in the study. VTE was associated with markers of disease severity including history of VTE before pregnancy (6/17 VTE vs 8/182 no-VTE, p<0.001), ACS during pregnancy (7/17 VTE vs 19/182 no-VTE, p<0.001), painful events requiring acute care during pregnancy (16/17 VTE vs 119/182 no-VTE, p=0.015) and hydroxyurea use before pregnancy (10/17 VTE vs 35/184 no-VTE, p<0.001). There was no difference in age or genotype.
VTE was associated with earlier delivery and c-section delivery (Table 1).
Conclusion
In this study, 7% of cohort pregnancies had VTE. This rate exceeds reports from administrative datasets. 1 This may be due to the acuity of patients cared for at our centers and/or reflect undercounting of VTE in administrative datasets. In pregnant people with SCD, VTE is associated with VTE history, intrapartum painful events and ACS. Interventions to reduce acute events in pregnancy may help reduce VTE risk. While AC can be beneficial in preventing thrombotic events, careful assessment is essential to manage bleeding risks. Prospective data is needed to define the subpopulation of pregnant SCD patients who would benefit from AC prophylaxis antenatally.
Disclosures
Kuo:Vertex Pharmaceuticals: Consultancy; Agios Pharmaceuticals: Consultancy, Research Funding; Alexion Pharmaceuticals: Consultancy; Bristol Myers Squibb: Consultancy, Honoraria; Forma Therapeutics: Consultancy; Pfizer: Consultancy; Bioverativ/Sanofi/Sangamo: Membership on an entity's Board of Directors or advisory committees; Novo/Nordisk: Consultancy, Honoraria. Lanzkron:Pfizer: Current equity holder in publicly-traded company; Teva Pharmaceutical Industries: Current equity holder in publicly-traded company; Novo Nordisk: Consultancy; Bluebird Bio: Consultancy; Shire: Research Funding; Novartis: Research Funding; Imara/Enliven Therapeutics: Research Funding; Global Blood Therapeutics: Research Funding. Pecker:Novo Nordisk: Consultancy; Global Blood Therapeutics: Consultancy; Alexion: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal